Joseph R. Anticaglia MD
Medical Advisory Board
Every year millions of infants (6% of worldwide births) are born with serious birth defects. In the United States, birth defects are the leading cause of infant mortality accounting for one in every 5 infant deaths. They afflict about three per cent of the babies born in the US.
Scientists have been struggling for many decades to treat and cure hereditary diseases with little to no success. We all have defective genes in our cells, and at times one or both parents pass down faulty genes to their child causing a hereditary disease. It’s often described as “something that runs in the family.”
At other times, parents may show no evidence of defective genes, yet the child is born with a genetic disease. The genetic disease is not passed down from parent to child. The unknown causes of genetic diseases may happen as much as 50% of the time. Spontaneous mutations and environmental factors may contribute to this situation.
Hereditary diseases can rob patients of their sight, trouble with hearing, difficulty with breathing, a healthy heart and cause blood diseases such as sickle cell anemia and thalassemia. Children, parents and adults have renewed hope because of advances made in genetic research and the dawn of a new specialty.
Gene Surgeons and the New Specialty
There are approximately 5,000 to 8,000 inherited disorders caused by a defect in one single gene (monogenic disorders). This single, genetic defect or mutation has been responsible for diseases that include cystic fibrosis, sickle anemia, thalassemia, Huntington’s disease and Duchenne muscular dystrophy.
The new specialty, Gene Surgery, is leaping across the scientific world. The surgeons who perform gene editing procedures don’t make skin incisions. They use “molecular scalpels” to change an organism’s DNA.
Gene Surgeons are on the verge of curing genetic diseases and reducing the emotional trauma felt by patents and parents. This has been made possible thanks to CRISPR-Cas9 and the work of 20220 Nobel Prize winners, Jennifer Doudna and Emmanuelle Charpentier.
CRISPR-Cas9
Gene Surgeons use CRISPR-Cas9 gene editing to target, delete, repair or replace a faulty gene. As noted in an earlier post there are…
Two Main Components of the CRISPR-Cas9 system:
- gRNA — guide ribonucleic acid
- Cas9 — is the cutting protein
Guide RNA’s role is to recognize and target the section of DNA to be edited. Guide RNA becomes attached to Cas9 and together they navigate toward the targeted DNA in the cell.
Cas9 is the molecular scalpel used to cut—cleave and degrade an unwanted section (or sequence) of DNA with its faulty gene at a specific site, in a specific way.

CRISPR surgeons also target two types of cells to gene edit:
- Somatic cell
- Germline cell
A somatic cell is any cell in a person’s body apart from reproductive cells. The eggs and sperm are reproductive cells. Somatic cell gene therapy removes faulty genes and inserts “good” genes into these cells. The insertion of good genes do not get passed down from parent to offspring. This form of treatment is relatively uncontroversial.
Germline cell gene therapy involves the insertion of “good” genes into reproductive cells. The procedure changes the genetic makeup of the cells and the lifetime hereditary changes will pass to future generations.
Gene Editing Controversy
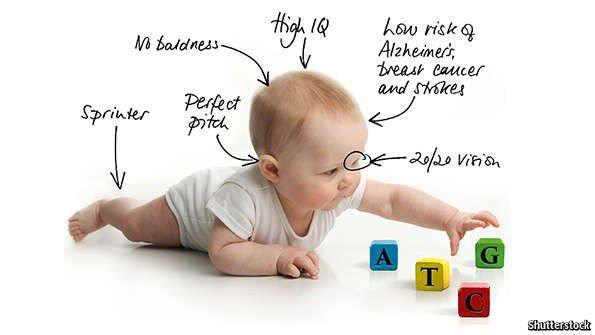
The use of CRISPR-Cas9 to manipulate the germline has a tremendous upside to treat patients’ diseases. This technology, in addition, has the potential to enhance desirable traits, such as vision hearing or I. Q.
However, a worrisome concern is that this nascent technology is not foolproof. There is off target effects wherein Cas9 attaches to off-targeted sites with unforeseen consequences. Such gene editing has mistakenly deleted and rearranged large sections of DNA. Studies warn that CRISPR gene edited cells can trigger cancer.
Dr. He Jiankui Lulu and Nana
The Chinese gene editing researcher, He Jiankui provoked an international outcry after his announcement in November, 2018 that he created the first genetically edited babies. Dr. Jiankui announced he implanted CRISPR gene edited embryos into a mother’s womb to prevent the babies from becoming infected with H. I. V. He noted that the mother delivered healthy twin girls known by their pseudonyms Lulu and Nana.
He was initially lionized, but soon after was demonized. About a year after his announcement in 2018, the Chines court found Dr Jiankui guilty of: “illegal medical practices” and sentenced him to three years in prison. The court also fined him close to $450,000. The scientific community heaped criticism on Dr. Jiankui.
Dr. Jennifer Doudna asked the scientific community to pause and discuss the ethical and social implications of CRISPR. The director of the National Institute of Health, Dr. Francis Collins, said the experiment was “profoundly disturbing.” Joyce Harper of the University College said Dr. Jiankui’s experimentation with human embryos was “premature and dangerous.”
Yellow Light for Gene Surgeons
CRISPR-Cas9 has given hope to people with genetic and other diseases. There’s also the possibility of enhancing one’s natural traits. CRISPR has the making of being a miracle worker.
But there are warning signs that scientists just don’t know enough about the long term consequences of this technology. The viewpoint among many gene editing scientists is to drive forward cautiously and along the way, pump the brakes. Gene drive can cause untold havoc.
References
- Mia Georgiou; Meet Lulu and Nana, the world’s first CRISPR genome-edited babies; Education Technology, September 30, 2020
- CDC Birth Defects Data & Statistics
- Libo, Ingrid, Zhaurova, Kira; Birth Defects: Causes and Statistics; Nature Education, 2008
- Ashok M. Patil; Gene editing and therapy — A new tool: CRISPR/Cas9; Al Ameen J Med Sci, 2019; 12(3):
- Joseph R. Anticaglia, MD; A 21st Century Scientific Revolution? CRISPR-Case 9; Doctor’s Column, 2021
- Monogenetic Disorders (Single Abnormal Gene); Virtual Genetics Education Centre; University of Leicester
- H. T. Greely; Ethical Issues in the “New” Genetics; International Encyclopedia of the Social and Behavioral Sciences, 2001

This article is intended solely as a learning experience. Please consult your physician for diagnostic and treatment options.

